Author: Harmeet Kaur Bhatia, Harmanjit Singh,1 Nipunjot Grewal,2 and Navreet Kaur Natt3
Abstract
Hepatitis C currently infects more than 170 million people around the world, leading to significant morbidity and mortality. The current standard of care for HCV infection, including one of the two protease inhibitors, telaprevir or boceprevir, for 12-32 weeks, along with pegylated interferon alfa-2a (PEG-IFN-α) and ribavirin for up to 48 weeks, is unsatisfactory in many cases, either because of lack of efficacy or because of treatment-related adverse effects. There is an urgent need of new drugs with improved efficacy as well as a safety profile. Sofosbuvir, a recently approved nucleotide analog, is a highly potent inhibitor of the NS5B polymerase in the Hepatitis C virus (HCV), and has shown high efficacy in combination with several other drugs, with and without PEG-INF, against HCV. It offers many advantages due to its high potency, low side effects, oral administration, and high barrier to resistance. The efficacy and safety were demonstrated in many large and well-designed phase 2 and phase 3 clinical trials like NEUTRINO, PROTON, ELECTRON, ATOMIC, COSMOS, FUSION, FISSION, NUCLEAR, POSITRON, and the like. It is generally well-tolerated. Adverse events that occurred include: Headache, insomnia, fatigue, nausea, dizziness, pruritis, upper respiratory tract infections, rash, back pain, grade 1 anemia, and grade 4 lymphopenia; however, the exact safety profile can only be judged when this drug is actually used on a large scale.
Keywords: Hepatitis C, NS5B polymerase, sofosbuvir, SVR
INTRODUCTION
Hepatitis C, caused by various genotypes of the Hepatitis C virus (HCV), currently infects more than 170 million people around the world. The infection may lead to chronic hepatitis, decompensated cirrhosis, and hepatocellular carcinoma, causing as many as 350,000 deaths per year. The majority of the cases are caused by HCV genotypes 1 (70%) and 4, less frequently by types 2 and 3. The current standard of care for infection by HCV genotype-1 includes, one of the two protease inhibitors, telaprevir or boceprevir, for 12 – 32 weeks, along with pegylated interferon alfa-2a (PEG-IFN-α) and ribavirin for up to 48 weeks.The treatment duration is guided by on-treatment response, allowing for shortening of the duration to 24 – 28 weeks in patients without cirrhosis, who show clearance of HCV RNA within the first eight weeks of therapy For genotypes 2 and 3, the recommended treatment is PEG-IFN-α and ribavirin for 24 weeks
Sustained virological response (SVR), defined as undetectable HCV RNA in the serum for 24 weeks, after the end of treatment, is only seen in 60 – 80% of previously untreated patients and 55 – 60% of previously treated patients with HCV genotypes 2 and 3. Higher SVR is seen in genotype 1 infections with triple therapy, in the range of 69 – 88% in previously untreated and 59 – 68% in previously treated patients. SVR is associated with improved outcomes in the form of reduction in the rate of hepatocellular carcinoma (HCC) and liver decompensation, and improved survival; patients who achieve SVR are considered to be cured.
In addition to the patient population that is not cured by the available regimens, is the burden of numerous patients who go untreated due to contraindications (advanced hepatic disease, autoimmune disease, and psychiatric illness) or refusal to receive interferons, as well as poor compliance or discontinuation of therapy due to adverse effects (fatigue, headache, fever, cytopenia, autoimmune disorders, insomnia, and depression). Other downsides of interferons include their need to be injected and the long duration of treatment. Although regimens containing protease inhibitors have resulted in higher SVR and shorter duration of treatment, their limitations include a low genetic barrier to resistance, more side effects, complex medication regimens, and a potential for drug interactions. In patients who do not achieve SVR with the current treatment options as well as those that go untreated, newer options are required. Researchers are now evaluating the combination of two or more antiviral agents, with separate targets for possible interferon-free regimens with higher SVR and shorter duration of treatment.
WHAT IS SOFOSBUVIR?
Sofosbuvir is a new drug candidate for hepatitis C treatment, with the chemical name L-Alanine, N-[[P(S),2′R]-2′-deoxy-2′-fluoro-2′-methyl-P-phenyl-5′-uridylyl]-, 1-methylethyl ester and a molecular formula of C22H29FN3O9P. Previously known as PS-7977 or GS-7977, it has shown promising results in numerous in vitro studies against all the genotypes of HCV. It is a nucleotide analog that is a highly potent inhibitor of the NS5B polymerase in HCV. This drug has shown high efficacy in combination with several other drugs with and without PEG-INF, against HCV. Sofosbuvir is of special interest among the directly acting antiviral drugs under development, due to its high potency, low side effects, oral administration, and high barrier to resistance.
MECHANISM OF ACTION
Hepatitis C virus is a single-stranded RNA virus, and its open-reading frame encodes ten structural proteins (viral capsid and envelope) and non-structural proteins (required for viral replication). NS5B is one of the non-structural proteins essential for viral RNA replication, and has been found to be a valuable target for directly acting antiviral agents (DAAs). The uridine nucleotide analog sofosbuvir is a phosphoramidate prodrug that has to be triphosphorylated within the cells to produce its action. The required enzymes for its activation are present in the human hepatic cells, therefore, it is converted to its active metabolite during the first-pass metabolism, directly at the desired site of action: The liver. The metabolic pathway for activation of the prodrug is shown in Figure 1. This analog then mimics the physiological nucleotide and competitively blocks the NS5B polymerase, thus inhibiting the HCV-RNA synthesis by RNA chain termination. The catalytic site of the enzyme is also highly conserved across all the HCV genotypes, accounting for pan-genotypic efficacy of sofosbuvir.
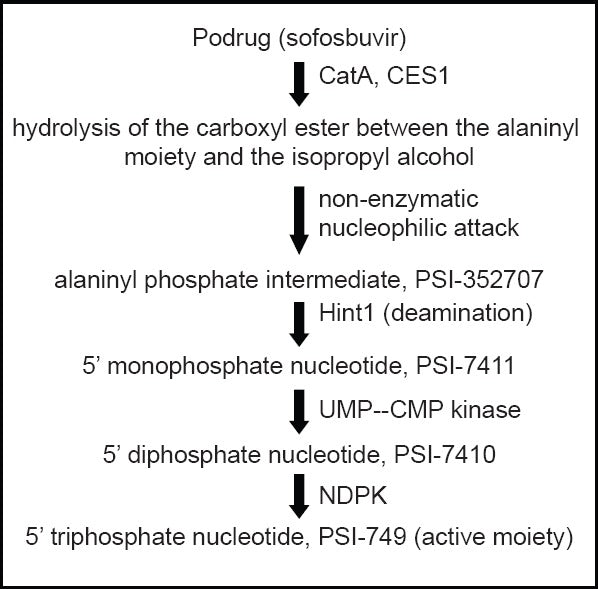
Activation of sofosbuvir in liver. CatA -human cathepsin A; CES1- carboxylesterase 1; Hint1 – histidine triad nucleotide-binding protein 1; NDPK – nucleoside diphosphate kinase; UMP–CMP kinase-uridine monophosphate–cytidine monophosphate kinas
PHARMACOKINETICS OF SOFOSBUVIR
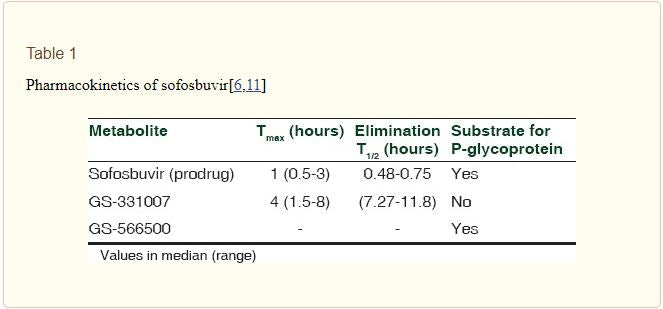
Several studies on the pharmacokinetics of sofosbuvir have been carried out and some are ongoing at present – alone and in combination with other drugs. Sofosbuvir has a beneficial pharmacokinetic profile, being effective orally as a single daily dose; this is likely to improve the compliance compared to protease inhibitors (with multiple oral daily doses), and PEG-IFN (parenteral administration). Absorption and elimination were observed after single and multiple doses of sofosbuvir, the results are shown in Table 1. The metabolic activation of the prodrug takes place by the enzymes present in the human liver [Figure 1]. A systemic exposure of >90% is due to the metabolite GS-331007 (previously PSI-6206), which also has a longer tmax and elimination t1/2 than sofosbuvir.
The effect of hepatic impairment was studied in a seven-day treatment with sofosbuvir in 17 patients with moderate-to-severe HCV-related hepatic impairment compared to eight non-cirrhotic patients infected with HCV. There was no significant difference in the half-life with or without hepatic impairment. The Cmax was 80% higher, and the AUC was 130% higher in subjects with hepatic impairment, while the viral decline was less pronounced. The safety profile was good in all the patients, thus suggesting that no dosage or interval modification was required in patients with moderate-to-severe hepatic impairment.
The effect of renal impairment on the pharmacokinetics of sofosbuvir has also been studied with a single 400 mg dose, in patients with varying degrees of renal impairment. The AUC of an inactive nucleotide metabolite, PSI-6206, is increased by 56% in mild, 90% in moderate, and 456% in severe renal impairment subjects, compared to normal subjects. Dosage or interval modifications are thus suggested in patients with moderate-to-severe renal impairment. Furthermore, 15% of sofosbuvir and 53% of PSI-6206 have been extracted by hemodialysis in patients with end-stage renal disease; dosage modifications will be recommended in this group of patients.
EFFICACY
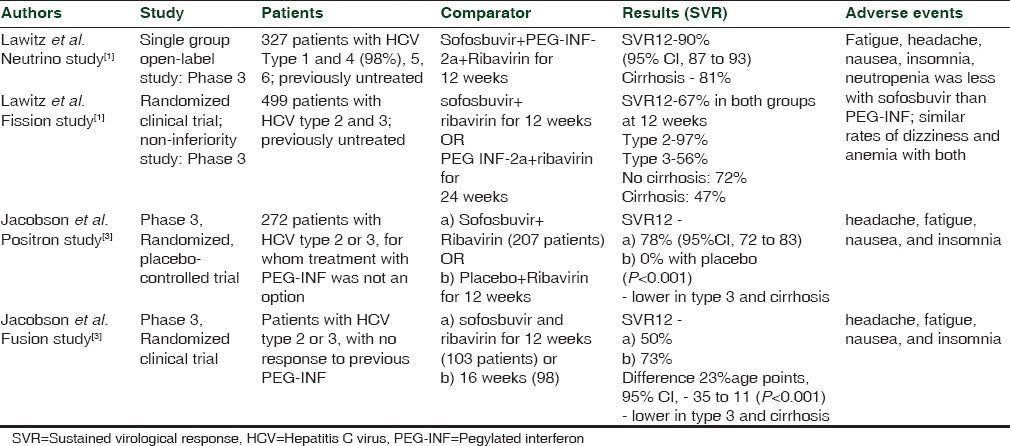
The efficacy and safety of sofosbuvir in patients with different HCV genotypes and with various combinations of drugs have been tested in numerous clinical trials. A dose of 400 mg of sofosbuvir has been found to be most effective, with treatment durations ranging from 12 to 24 weeks, in various combinations of PEG-IFN and ribavirin in phase 2 clinical trials [Table 2]. The NEUTRINO study found SVR to be 90% (95% CI, 87 to 93) 12 weeks after therapy with sofosbuvir + PEG-INF + ribavirin; this was found to be superior to the adjusted historical response rate of 60% (P < 0.001). Similar positive results have been found in numerous phase 3 clinical trials [Table 3]. Furthermore, recent phase 1 and 2 studies of sofosbuvir in combination with other DAAs have also shown promising results [Table 2].
Table 2
Phase 1 and 2 clinical studies involving sofosbuvir
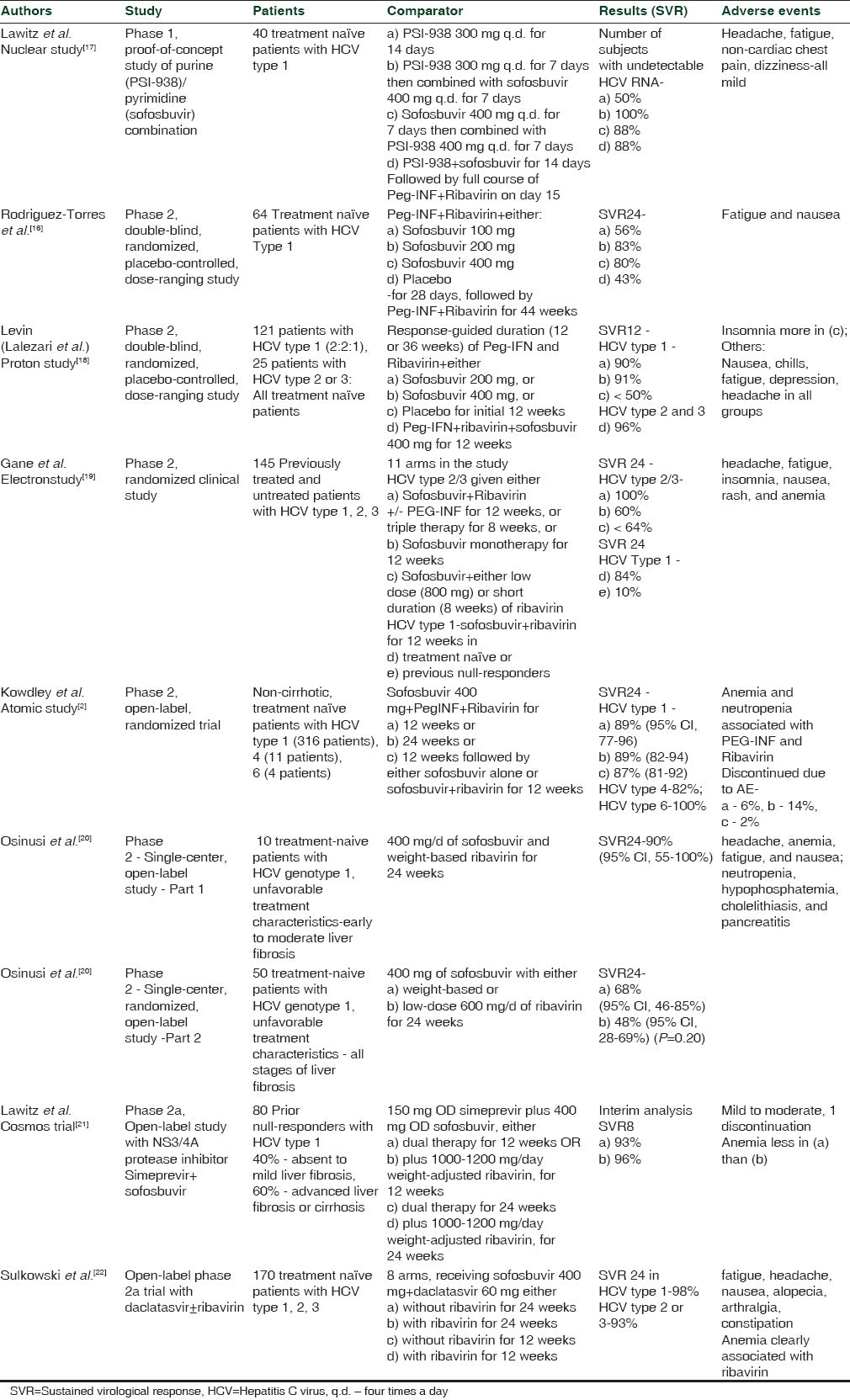
Table 3
Phase 3 clinical trials involving sofosbuvir
ONGOING TRIALS
Numerous studies are either recruiting or active at present, evaluating sofosbuvir in different populations of patients and with different drug combinations for varying durations. Sofosbuvir plus ribavirin all-oral combinations are being assessed in specific populations such as those having HCV genotype 4, in patients with renal insufficiency, concomitant HIV, hepatocellular carcinoma pre-transplantation, chronic HCV with cirrhosis and portal hypertension, or recurrent chronic HCV post liver transplant. The combination of sofosbuvir, ribavirin, plus PEG-INF is being evaluated in some ongoing trials including the FISSION and NEUTRINO trials, as well as in patients with aggressive post-transplant hepatitis. Several studies are underway evaluating the fixed dose combination of sofosbuvir 400 mg + ledipasvir (NS5A inhibitor, GS-5885) 90 mg, in the different HCV genotypes. Other drugs being tested in combination with sofosbuvir in ongoing trials include: GS-9669 (NS5B non-nucleoside thumb site II inhibitor) and GS-5816 (second-generation NS5A inhibitor).
RESISTANCE
DAAs, including NS3/4 A protease inhibitors and NS5A inhibitors, have shown beneficial results in the treatment of HCV; however, this is at the cost of rapidly emerging resistance, resulting in either a virological breakthrough during the treatment or a relapse thereafter. The high genetic barrier to resistance to sofosbuvir distinguishes it from other members in this group.
Cross-resistance studies have been conducted using panels of replicons with mutations in the NS3/4A protease, NS5A, and NS5B, which remained susceptible to sofosbuvir (except for HCV type 1b S282T), thus indicating that sofosbuvir can be combined with other directly acting antiviral agents. It has been suggested that additional mutations with amino acids change in both the finger and palm domains (T179A, M289L, and I293L) and are required to compensate for poor HCV fitness, resulting from S282T mutation, in order to confer resistance to sofosbuvir. The S282T mutation has so far only been detected in one patient, with HCV type 2b; a relapse has been seen in this patient after sofosbuvir monotherapy. Genotype or subtype-specific resistance has not been seen with sofosbuvir.
In the clinical studies, although relapse leading to treatment failure was seen in a few patients, no virological resistance was detected in these patients receiving sofosbuvir 400 mg monotherapy or in combination with either ribavirin, PEG-INF or both. One patient in the FISSION trial had a virological breakthrough, but this was most probably the result of non-compliance, as the levels of sofosbuvir were not detectable in the patient. The presence of a high barrier of resistance to sofosbuvir is a result of the highly conserved nature of the NS5B polymerase; variants in the active site of this enzyme result in the detrimental condition of the virus.
ADVERSE EFFECT PROFILE OF SOFOSFUVIR
Sofosbuvir has shown a good safety profile in clinical trials; a small decrease in the Hb levels (0.54 mg/dl) and reduction in the cumulative events in comparison to interferon-containing regimens is seen. Common adverse events observed include: Headache, insomnia, fatigue, nausea, dizziness, pruritis, upper respiratory tract infections, rash, back pain, grade 1 anemia, and grade 4 lymphopenia. No neutropenia, thrombocytopenia, or any serious adverse events are associated with sofosbuvir treatment. In the monotherapy treatment groups, nausea and fatigue seemed to be the only adverse events possibly correlated to sofosbuvir. An overall improved tolerability was seen with sofosbuvir compared to the interferon-based regimens.
DRUG INTERACTIONS OF SOFOSBUVIR
Many patients with HCV have concomitant illnesses such as HIV requiring anti-retroviral therapy, or hepatocellular carcinoma/decompensated liver disease requiring liver transplants along with immunosuppressant medication. Thus, it is very important to study the possible drug interactions that may occur in these patients, who also require treatment of HCV. Studies have shown no clinically significant interactions between sofosbuvir and the following drugs: Cyclosporine, tacrolimus, methadone, efavirenz, rilpivirine, darunavir/ritonavir, raltegravir, and tenofovir. No dose adjustments are required in patients receiving these drugs along with sofosbuvir. As sofosbuvir is being tried for all-oral regimens combined with other directly acting antiviral agents, interactions with these drugs have also been studied. No clinically significant interactions have been found between sofosbuvir and daclatasvir, ledipasvir, or GS-9669. A 54-year-old liver transplant recipient with HCV type 1b and severe recurrent cholestatic hepatitis was given daclatasvir (HCV NS5A inhibitor) plus sofosbuvir for 24 weeks; SVR at 36 was achieved, and the level and dose of tacrolimus remained stable in this patient.
CURRENT STATUS
The US FDA has recently (6 December, 2013) approved sofosbuvir under the brand name Sovaldi for the treatment chronic HCV infection under a breakthrough therapy designation, because it has shown a substantial improvement over the other available therapies. The most interesting feature of this approval lies in the fact that this drug can be administered without the need of interferon therapy. On the basis of the type of HCV infection, the treatment regimen may include sofosbuvir and ribavirin/sofosbuvir, ribavirin, and Peg-interferon-alfa. Earlier it was under the FDA’s priority review program (an expedited review of drugs useful for serious conditions, which, after their approval, would provide significant improvement in safety or effectiveness). Positive results from major clinical trials, plus a demonstration of efficacy in patients who cannot tolerate interferon-based regimens and in patients with liver cancer undergoing liver transplantation, make this drug a valuable and very useful therapy for these patient populations.
CONCLUSION
From the above discussion, it seems that sofosbuvir is a promising therapy for chronic HCV infection, as it offers several advantages over the existing therapies, particularly in dealing with patients with decompensated liver disease and patients who cannot tolerate interferon-containing therapies. On account of its excellent performance in clinical trials, this drug has got FDA approval on 6 December, 2013, under the breakthrough therapy designation. This drug is effective against all HCV genotypes, has a better safety profile, and low risk of development of resistance; however, careful clinical use and monitoring is still essential, to gather more data on this drug. Large post-marketing studies, including pharmacoepidemiological and pharmacovigilance studies, can solve many unanswered questions for the future of this novel drug. As of now, sofosbuvir is among the most promising agents available for the treatment of chronic HCV infection.
REFERENCES
1. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. [PubMed]
2. Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): An open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–7. [PubMed]
3. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–77. [PubMed]
4. Zeng QL, Zhang JY, Zhang Z, Wang LF, Wang FS. Sofosbuvir and ABT-450: Terminator of hepatitis C virus? World J Gastroenterol. 2013;19:3199–206. [PMC free article] [PubMed]
5. Herbst DA, Jr, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22:527–36. [PubMed]
6. Gentile I, Borgia F, Buonomo AR, Castaldo G, Borgia G. A novel promising therapeutic option against hepatitis C virus: An oral nucleotide NS5B polymerase inhibitor sofosbuvir. Curr Med Chem. 2013;20:3733–42. [PubMed]
7. Soriano V, Vispo E, de Mendoza C, Labarga P, Fernandez-Montero JV, Poveda E, et al. Hepatitis C therapy with HCV NS5B polymerase inhibitors. Expert Opin Pharmacother. 2013;14:1161–70. [PubMed]
8. Statement on a Nonproprietary Name Adopted by the USAN Council. [Last accessed on 2013 Oct 2]. Available from: http://www-ama-assn-org/resources/doc/usan/nabiximols.pdf .
9. Lam AM, Espiritu C, Bansal S, MicolochickSteuer HM, Niu C, Zennou V, et al. Genotype and subtype pro-ling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother. 2012;56:3359–68. [PMC free article] [PubMed]
10. Murakami E, Tolstykh T, Bao H, Niu C, Steuer HM, Bao D, et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010;285:34337–47. [PMC free article] [PubMed]
11. Drug Interactions with Investigational Hepatitis C Nucleotide Inhibitors. Sofosbuvir. [Last accessed on 2013 Sep 28]. Available from: http://www.hcvdruginfo.ca/downloads/Hepatitis%20C-int_new%20nucleotide%20inhibitors.pdf .
12. An Open-Label Study of Sofosbuvir/Ledipasvir Fixed-Dose Combination in subjects with nosocomial genotype 1 HCV infection. [Last accessed on 2013 Nov 11]. Available from: http://clinicaltrials.gov/ct2/show/NCT01924949 .
13. Open-Labeled Study of PSI-7977 and RBV with and without PEG-IFN in Treatment-naÏve Patients with HCV GT2 or GT3. [Last accessed on 2013 Oct 5]. Available from: http://clinicaltrials.gov/show/NCT01260350 .
14. Sofosbuvir Containing Regimens for the Treatment of Chronic HCV Infection in Subjects with Chronic Genotype 1, 2, or 3 HCV Infection. [Last accessed on 2013 Oct 12]. Available from: http://clinicaltrials.gov/ct2/show/NCT01826981 .
15. Safety and Efficacy of Sofosbuvir/GS-5885 Fixed-dose Combination ± Ribavirin for the Treatment of HCV (ION-2) [Last accessed on 2013 Oct 16]. Available from: http://clinicaltrials.gov/show/NCT01768286 .
16. Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, et al. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: A randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–8. [PubMed]
17. Lawitz E, Rodriguez-Torres M, Denning J, Cornpropst MT, Clemons D, McNair L, et al. Once daily dual-nucleotide combination of PSI-938 and PSI-7977 provides 94%HCVRNA < L OD at day 14:First purine/pyrimidine clinical combination data(THENUCLEARSTUDY). Proceedings of the 46th Annual Meeting of the European Association for the Study of the Liver. EASL. 2013 Apr
18. Levin J. EASL 46th Annual Meeting. PROTON Study: PSI-7977 QD with PEG/RBV: 12-week Safety, RVR, cEVR, and SVR12 in Treatment-naÏve Patients with HCV GT2 or GT3. [Last accessed on 2013 Oct 15]. Available from: Http://www.natap.org/2011/EASL/EASL_22.htm. http://clinicaltrials.gov/show/NCT01768286 .
19. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. [PubMed]
20. Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: A randomized clinical trial. JAMA. 2013;310:804–11. [PMC free article] [PubMed]
21. Highleyman L. DDW 2013: Interferon-free Simeprevir + Sofosbuvir Suppresses Hepatitis C with or without Ribavirin. 2013. May, [Last accessed on 2013 Oct 18]. Available from: http://www.hivandhepatitis.com/hepatitis-c/hepatitis-c-topics/hcv-treatment/4126-ddw-2013-interferon-free-simeprevir-sofosbuvir-suppresses-hepatitis-c-with-or-withoutribavirin .
22. Levin J. High Rate of Sustained Virologic Response with the All-oral Combination of Daclatasvir (NS5A Inhibitor) Plus Sofosbuvir (Nucleotide NS5B Inhibitor), with or without Ribavirin, in Treatment-naive Patients Chronically Infected with HCV GT 1, 2, or 3. Presented at 63rdAnnual Meeting of the American Association for the Study ofLiver diseases Boston, MA Nov 9-12 2012. [Last accessed on2013Oct 15]. Available from: Http://www.natap.org/2012/AASLD/AASLD_06.htm .
23. Highlyman L. Polymerase Inhibitor PSI-7977 Works with Interferon or Companion Drug. Coverage of the 46thAnnual Meeting of the European Association for the Study of the Liver. 2011. Apr, [Last accessed on 2013 Oct 10]. Available from: http://www.hivandhepatitis.com/2011_ conference/easl2011/docs/0404_2010_ d.html .
24. Levin J. GS-7977 and HIV ARTs PK – No Clinically Significant Pharmacokinetic Interactions between Sofosbuvir (GS-7977) and HIV Antiretro virals Atripla, Rilpivirine, Darunavir/Ritonavir, or Raltegravir in Healthy Volunteers. AASLD- 63rd Annual Meeting of the American Association for the Study of Liver Diseases. 2012. Nov, [Last accessed on 2013 Oct 5]. Available from: http://www.natap.org/2012/AASLD/AASLD_64.htm .
25. Data from Phase 3 Studies of Gilead’s Sofosbuvir for Hepatitis C to be Presented at 48thAnnual EASL Meeting; Findings Published Online in The New England Journal of Medicine. 2013. Apr, [Last accessed on 2013 Oct 10]. Available from: http://www.gilead.com/news/press-releases/2013/4/data-from-phase-3-studies-ofgileads-sofosbuvir-for-hepatitis-c .
26. Fontana RJ, Hughes EA, Bifano M, Appelman H, Dimitrova D, Hindes R, et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am J Transplant. 2013;13:1601–5. [PubMed]
27. FDA Approves Sovaldi for Chronic Hepatitis C. [Last accessed on 2012 Dec 6]. Available from: http://www.fda.gov/News Events/Newsroom/PressAnnouncements/ucm377888.htm .
- [Disclaimer]The Site is operated by GDMeds and all rights thereto are owned and reserved by GDMeds. The contents and works on these pages compiled by GDMeds are subject to copyright law. Copying, processing, distribution and any kind of use outside the limits of copyright law require the written consent of GDMeds In case the content is not created by GDMeds the copyrights of third parties are being observed. However, if a user becomes aware of a copyright infringement, GDMeds asks the user for notification. Upon notification of such violations, GDMeds will remove the content immediately.
- [免责声明]本网站由印度极得美运营。印度极得美拥有和保留一切权利。印度极得美的网页内容及文档受版权法保护。复制、加工、传播及任何超出版权法限制的任何使用行为均必须得到印度极得美书面许可。如相关内容如非由印度极得美创作,需遵守第三方的版权,特别是标示为第三方内容的。如用户发现侵犯版权的行为,请通知极得美。一旦收到违反通知,极得美将立刻移除相关内容。
- [отказ]Сайт управляется GDMeds, и все права на него принадлежат и зарезервированы GDMeds. Содержание и работы на этих страницах, составленные GDMeds, защищены законом об авторском праве. Копирование, обработка, распространение и любое использование вне пределов авторского права требуют письменного согласия GDMeds. Если контент не создан GDMeds, то соблюдаются авторские права третьих лиц. Однако, если пользователь узнает о нарушении авторских прав, GDMeds запрашивает у пользователя уведомление. После уведомления о таких нарушениях GDMeds немедленно удалит контент.